胺的分类
胺是一类重要的有机化合物。它们广泛存在于植物和动物中。它们存在于蛋白质、维生素、生物碱、激素等中。合成的例子包括聚合物、药物、染料等。这些胺被广泛使用。例如,奎宁是一种重要的抗疟疾药物,肾上腺素和麻黄碱用于提高血压,诺卡因在牙科中用作麻醉剂,可待因用作镇痛剂(作为止痛药)。 Benadryl用作抗组胺药。季铵盐用作表面活性剂。
Amines are considered to be derivatives of ammonia in which one, two, or all three hydrogen atoms are replaced by an alkyl or aryl group.
胺的分类
胺被分解为伯 (1°)、仲 (2°) 或叔 (3°),因为氨分子中的一个、两个或三个氢被氨分子中的烷基或芳基取代。然而,如果氨的一个氢原子被烷基 (R) 或芳基 (Ar) 取代,我们会得到 RNH 或 ArNH 2 。然而,如果 RNH 的氨 H 原子的两个 H 原子被另一个烷基 (R) 或 Ar 基团取代,我们采用 R-NH-R,即 2° 胺。并且烷基或芳基可以相同或不同。
氨的所有三个 H 原子或 R-NH-R 的另一个 H 被烷基(素数高)或芳基取代,得到 Rn(素数)素数,它可以是 3° 胺 (R”) 相等或不相等。 R 或 R')。
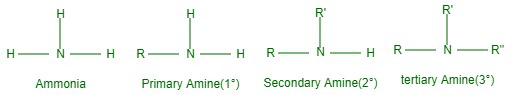
伯胺、仲胺和叔胺中的特征基团是氨基、亚氨基和叔氮。
除了这三种胺之外,还有一类称为季铵磅的化合物。这些化合物可以被认为是铵盐的衍生物,其中所有四个 H 原子都由烷基或芳基保持。例如四甲基碘化铵、四甲基氯化铵、四甲基四甲基铵甲基氢氧化铵、四甲基溴化铵等。
胺类可进一步分为两类:
- 脂肪胺:氮原子直接与一个或多个烷基键合的胺称为脂肪胺。例如,甲胺(1°)、二甲胺(2°)和三甲胺(3°)。
- 芳基烷基胺或侧链取代胺:其中氮原子连接到芳环侧链的胺称为烷基胺。例如,苄胺(1°)、二苄胺(2°)和三苄胺(3°)。
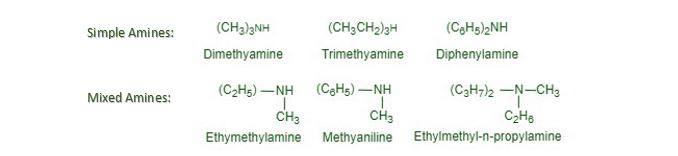
简单和混合胺。根据与氮原子连接的所有烷基或芳基相同或不同,仲胺和叔胺可分为简单胺或混合胺。例如,
胺的物理性质
- 溶解性:脂肪胺(1°、2° 和 3°)与水形成 H 键,因此可溶于水。然而,芳香胺不溶于水,主要是由于烃。
- 沸点- 1° 胺的沸点高于 3° 胺,因为存在两个直接连接到 N 上的 H 原子,导致 1° 胺中的 H 键更高。
胺的基本字符
- 脂肪胺
- 所有脂肪胺的碱性都比氨强。
- 在水溶液中,甲胺的碱度顺序为 (CH₂)₂NH > CH₂NH₂ > (CH₂), N (fe 2° 1 > 3º),但对于乙胺和所有其他高级胺,碱度顺序为 R₂NH > R₂N > RNH。跟随。 (即 2° > 3° > 1°)。
- 气态的碱度顺序是 3° > 2° > 1º 胺。
- K p的值越高或pK b的值越小,碱基越强。
- 芳香胺
- 所有芳香胺都是比氨弱的碱。
- 给电子基团如-CH 3、 -OCH 3、 -NH 2增加碱度,而吸电子物质如-NO 2 、-CN、-(卤素)降低胺的碱度。这些物质的影响在 p- 条件下比在 m- 条件下更明显。
- 无论取代基的给电子或吸电子性质如何,邻取代苯胺都是比苯胺弱的碱。这称为邻位效应,是由空间位阻引起的。
Aliphatic amines are more basic than aromatic amines. Like aromatic amines, the lone pair of electrons present on nitrogen participates in resonance and is therefore not available for donation, whereas it is available in aliphatic amines.
示例问题
问题 1:进行化学测试以区分乙胺和苯胺。
回答:
Ethylamine is a primary aliphatic amine whereas aniline is a primary aromatic amine. Both can be identified by the azo dye test. When aniline is treated with HNO, (NaNO₂+ dil. HCl) at 273K, followed by treatment with an alkaline solution of 2-naphthol, the orange color is obtained, but due to the formation of ethylamine. With N2 gas gives strong effervescence of 1 alcohol.
问题 2:进行化学测试以区分苯胺和 N-甲胺。
回答:
Aniline is a primary aromatic amine while N-methyl aniline is a secondary aromatic amine. The two can be separated by the carbylamine reaction. Upon mixing CHCl3 and KOH, aniline will give off an offensive odor due to the formation of phenyl carbylamine (phenyl isocyanide), while N-methyl aniline will not react.
问题 3:为什么芳香胺比苯更容易发生亲电取代?
回答:
Due to the strong activating group -NH₂, the electrons on the N-atom in aniline are denoted above the benzene ring. As a result, the electron density on the benzene ring increases as compared to benzene. Therefore, aniline becomes activated and electrophilic substitution occurs more easily in aniline.
问题 4:进行化学测试以区分苯胺和苄胺。
回答:
Aniline and benzylamine can be separated by azo dye test. Aniline reacts with NO2 at 273-278 K to form stable benzene diazonium chloride which gives orange color on reaction with an alkaline solution of β-naphthol. Whereas benzylamine does not give this test.
问题5:对甲氧基苯胺是比苯胺强的碱,但对硝基苯胺是比胺弱的碱。解释。
回答:
The methoxy group (-OCH3) is the electron freeing group and increases the electron density on the N atom. Therefore, it has a tendency to donate more electrons than aniline and thus is a stronger base than aniline. On the other hand, the nitro group is the electron withdrawing group and hence, the electron density on the nitrogen atom decreases. Consequently, p-nitroaniline is a weaker base than aniline.
问题6:加百列的邻苯二甲酰亚胺反应可以制备苯胺吗?
回答:
The Gabriel phthalimide reaction to aniline cannot be made because it requires treatment of potassium phthalimide with C6H5Cl or C6H5Br. Since aryl halides do not undergo nucleophilic substitution reactions under normal conditions, the reaction does not occur. Therefore, aniline cannot be prepared by this method.