有机反应机理的基本概念
有机化学是碳化合物的化学,除了碳的氧化物和金属碳酸盐。碳具有与许多其他元素,特别是与其他碳原子形成强键的不寻常特征,形成链和环,从而产生数百万个有机分子。由于这种独特的特性,碳化合物对于地球上生命的生存至关重要。蛋白质、DNA(脱氧核糖核酸)和其他复杂的有机物质为生物提供化学、结构或遗传功能。
化学方程式仅显示反应的起始和最终产物;它很少显示该过程是如何进行的。一些反应是通过中间体发生的,中间体可能会或可能不会被分离,具体取决于它们的稳定性。
Mechanism is the complete step-by-step description of the order in which bonds break and bonds form, to give the observed products.
有机反应中的电子运动
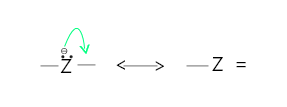
弯曲箭头用于描述化学过程中的电子传输。弯曲箭头是一个方便的指示,用于显示在反应过程中由电子再分布引起的键合变化。当两个原子共享一对电子时,就会产生共价连接。下面使用弯曲箭头描述了电子对传播的许多方法。箭头从电子对位移的点开始,到电子对移动的点结束。
- 电子对从 π 键移动到相邻键位置。
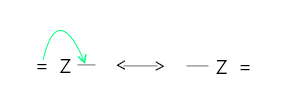
- 电子对从 π 键转移到相邻原子。

- 电子对从原子到相邻键位置的移动。
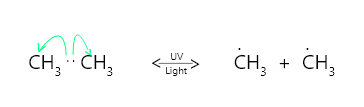
例如:乙烷在紫外光存在下反应时,由于电子转移,得到两个甲烷分子,如下图所示,
债券裂变
In any chemical reaction, when a reactant is converted into products one or more bonds in the reactant are broken and new bonds are formed. The process of breaking or cleavage of a covalent bond is known as bond fission.
现在,债券裂变以两种方式发生,如下所述,
均裂裂变
The symmetrical breaking of a covalent bond between two atoms such that each atom acquires one electron of the shared pair is called homolytic fission or homolysis.
这种裂变发生在有紫外线或高温的情况下。键的断裂导致自由基的形成。自由基是包含不成对电子的中性物质(原子或基团)。均裂裂变可以表示为,

单个电子的运动由半头弯曲箭头(鱼钩)表示。自由基具有短暂的存在,即它们是短暂的并且是高度反应性的。它们是顺磁性的。
通常,相同元素的两个原子或具有几乎相同电负性的两个原子之间的共价键以这种方式断裂。例如,通过均裂进行的有机反应称为自由基或同极性或非极性反应,因为这些反应发生在非极性溶剂中。均解通常在日光或紫外光存在下或在过氧化氢等催化剂存在下发生在气相中。
杂裂裂变
The unsymmetrical breaking of a covalent bond between two atoms such that the more electronegative atom acquires both the electrons of the shared pair is called heterolytic fission or heterolysis.
这种裂变在极性溶剂的存在下发生。键的断裂导致离子的形成。其中一个离子具有六重电子结构和称为阳离子的正电荷,而另一个离子具有具有至少一对孤对电子和负电荷的价八位元组,称为阴离子。
杂裂裂变可以表示为,

- 其中 B 比 A 更负电。
如果 A 比 B 更负电,则裂变将显示为,

形成的离子不稳定且具有反应性。异解的一个例子是,

碳原子具有六重电子和正电荷的物质称为碳正离子或碳鎓离子。碳正离子是缺电子的。在 C-Br 键中,溴原子比碳原子更负电,因此电子对在裂变时被溴原子保留。
但考虑一下反应:

在 CH 键中,碳原子比氢原子更具电负性,因此电子对在裂变时被碳原子保留。碳原子具有八位电子和负电荷的物质称为碳负离子。碳负离子是反应性的,因为它们不稳定。通过杂裂进行的有机反应称为离子或杂极性或简称为极性反应,因为这些反应发生在极性溶剂中。气态的杂解是不常见的。
What are Free Radicals?
An uncharged species which is electrically neutral and contains a single electron is called free radical.
Free radical is highly reactive and therefore has a transitory existence i.e. it is short-lived. Consider methyl radical in which carbon is sp3 hybridized and has a planar structure. H – C – H bond angle is 120 degrees The odd electron is in the p orbital which P is perpendicular to the plane of three C – H bonds (refer to the image shown below).
Carbon is electron deficient as it has only seven electrons in the valence shell.

The structure of the methyl radical is like that of the methyl cation, except there is an additional electron.
What are Reagents?
The reagent reacts with the substrate to give products. The reagent may be an electron-rich or electron-deficient chemical species that attacks the substrate during a chemical reaction.
The following are two types of important reagents.
- Electrophilic reagents or electrophiles: Electrophiles are electron-deficient species. They are either positively charged species like H, NO₂, etc. or molecules containing the central atoms having incomplete octet of electrons in their outermost orbit like BF, AICI, ZnCl₂, etc. Since electrophiles are electron-deficient, they accept a pair of electrons from donor atoms and thus they are electron loving reagents. All electrophiles are basically Lewis acids.
- Nucleophilic reagents or nucleophiles: Nucleophiles are electron-rich species. They are either negatively charged species like OH, CN, CT, Br etc. or molecules containing at least one lone pair of electrons on the central atom-like H₂O, NH₂, H₂S, R OH, R-NH₂, R-OR, etc. Since nucleophiles are electron-rich, they donate a pair of electrons to acceptor atoms and thus they are nucleus loving reagents. All nucleophiles are Lewis bases.
电致效应:共价键中的电子位移
分子共价键中电子的位移是由于存在合适的攻击试剂或由于原子或基态取代基的影响而发生的。
当试剂接近分子时会观察到临时电子位移,这种形式的电子位移被称为电聚效应。由分子中的原子或取代基引起的电子位移会产生键的永久极化。这种形式的电子位移以感应效应为例。
在某些条件下,共价键中的电子位移会导致键裂变。例如,均裂和异裂裂变。
感应效应
让我们首先了解两种重要的共价键类型,
- 非极性键:当相同元素的两个原子或具有相同电负性的两个原子之间建立共价键时,两个原子均等地共享成键电子对。这种类型的键本质上是非极性的。此类键的示例是 H - H、Cl - Cl、O = O 等。
- 极性键:当具有不同电负性值的不同元素的原子之间建立共价键时,键中的电子密度向电负性更强的原子移动。当电子密度发生变化时,形成极性共价键。例如,H – CI、H – OH、H 3 C – Cl 等。
考虑具有化学式 CH 3 – CH 2 – Cl 的氯乙烷。它被极化,使得一号碳获得正电荷(+δ),而氯获得负电荷(-δ)。从极性键的+δ指向-δ的箭头描绘了电子密度的变化。

C 1产生正电荷 (+δ),从附近的 C-C 键吸引电子密度。结果,一些正电荷(+δ 1 )出现在C 2上,其中+δ 1表示正电荷明显低于C 1上的正电荷。换句话说,极性 C - Cl 键导致相邻键变为极性。这种由相邻σ键极化引起的σ(σ)键极化称为感应效应。
这是一个长期的后果。这种影响也会持续到后续的键上,尽管它会随着碳链长度的增加而迅速减弱。粘合后,效果非常微不足道。
结果,电子对虽然被永久重新定位,但仍保留在相同的价壳中。
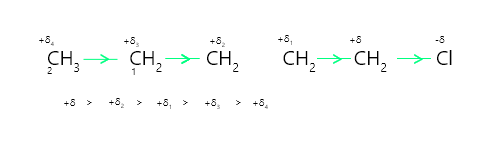
取代基去除或赋予连接碳原子电子密度的能力与感应效应有关。基于这种能力,取代基可以分类为:
- -I 效应(负感应效应)–具有高负电性或带有正电荷的原子或原子团是吸电子基团,据说这些基团具有 (-I) 效应。例如-F、-Cl、-Br、-I、-NO 2 、-CN、-COOH、-COOR、-SO 3 H等是吸电子基团。原子的电负性越高,-I 效应越大,例如 -I 效应按 F > Cl > Br > I 的顺序降低。带正电的原子或基团比中性原子或基团具有更大的 -I 效应,例如 - N + O 2比-NH 2具有更多的-I效应
- +I 效应(正感应效应)–带正电或带负电荷的原子或原子团是供电子基团,据说这些基团具有 (+I ) 效应。 Na、K、Mg、Zn等金属和-CH 3 、-CH 2 CH 3 、-CH(CH 3 ) 2等烷基是给电子基团。 CH 3 O-、C 2 H 5 O-等带负电的基团表现出强的+I效应。电负性较小的元素具有较大的 +I 效应,例如 Be > B > C。类似地,带负电的原子或基团比中性原子具有更大的 +I 效应,例如 CH 3 – CH 2 > CH 3 – CH 3 。
电磁效应
某些化学物质在非极性共价键中产生极性或提高极性共价键中的极性。这被称为电致效应。 

电磁效应是一种暂时的效应,但它通过诱导或增强具有许多键的底物的极性来帮助增加分子的反应性。
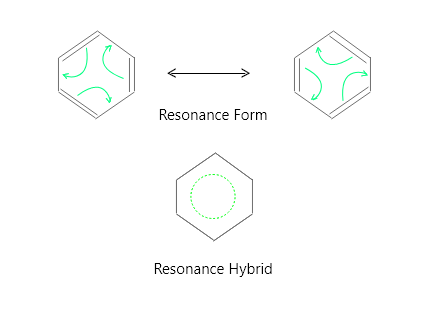
谐振
When a carboxylic acid loses a proton, the electron density is shared by both oxygen atoms – the electrons are delocalized. Delocalized electrons are not bound to a single atom or a link between two atoms. A compound having delocalized electrons is said to have resonance.
使用局部电子的两种结构称为共振贡献者、共振结构、共振形式或贡献共振结构。这些共振形式都不是羧酸根离子的正确结构。真实结构是两种结构的混合体,称为共振混合体,它用虚线表示,以证明电子是离域的。共振形式由双头箭头表示。

共振形式

共振混合
负电荷(电子)分布在两个氧原子上。每个氧原子带有一半的负电荷,使离子稳定。碳-氧键的键序为 1 1 / 2 ,这意味着它们介于单键和双键之间。只有当所有共享离域电子的原子都在同一平面内或附近时,电子离域才会发生,从而允许它们的 p 轨道有效地重叠。
Note: The only difference between the two resonance forms of carboxylate ion is the placement of their π electrons and lone-pairs; all of the atoms remain in the same spot.
简而言之,共振,也称为介观异构现象,是指化合物以两种或多种电子结构混合的状态存在的现象,每一种电子结构似乎都同样能够表达化合物的大部分属性,但都没有描述了所有的品质。
共振稳定:共振混合结构的能量低于任何有贡献的共振结构。真实结构(共振混合)与最低能量共振结构之间的能量差称为共振稳定能量,或简称为共振能量。共振能量随着关键贡献共振结构的数量增加而增加。
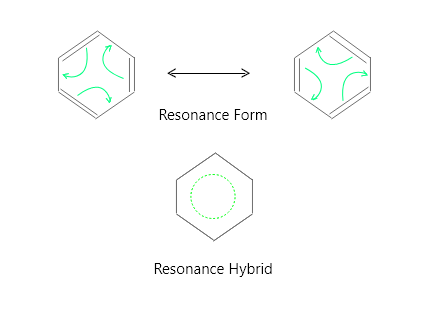
Resonance in Benzene

Structure of Benzene
- In the below two images, each benzene resonance form clearly demonstrates that the ring has six π electrons. The resonance shapes are only a handy technique to portray the π electrons; they do not represent any actual electron distribution. In benzene, for example, the link between C-1 and C-2 is neither a double bond as indicated in figure1 nor a single bond as shown in figure 2.
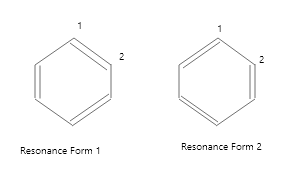
- It falls somewhere in the middle of the two resonance forms. The resonance hybrid, which is the average of the two resonance forms, is the true structure of benzene.

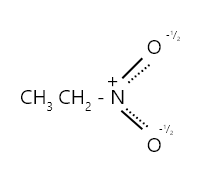
Resonance in Nitroethane

Resonance Form
- In the above image, the double bond in the 1st structure is the single bond and in the 2nd structure, it’s vice versa.
Resonance Hybrid
- The resonance hybrid shows that the two nitrogen-oxygen bonds are identical and the negative charge is shared by both oxygens. The p orbital of nitrogen overlaps the p orbital of each oxygen. In other words, the two electrons are shared by three atoms.
绘制共振结构的规则
- 只有电子移动;原子核从不移动,因此键角必须保持不变。
- 唯一可以传播的电子是 π 电子和孤对电子。
- 如果有的话,未配对电子的数量必须保持不变。大多数稳定的化合物没有不成对的电子,所有电子必须在所有共振结构中保持耦合。
- 能量最少的共振贡献者是最重要的。好的贡献通常会完成所有八位字节,尽可能多的键,以及尽可能少的电荷分离。负电荷在更多电负性元素(如 O、N 和 S)上更稳定。
- 当共振稳定用于使电荷在两个或多个原子上离域时,它尤其重要。
Electrons can be moved in one of the following ways –
- Move π electrons towards a positive charge or towards a π bond:

(movement of π electron toward a positive charge)
(movement of π electron toward a π bond)
- Move a lone pair of electrons towards an π bond:

- Move single non-bonding electron towards π bond:

根据电子转移,共振效应分为两种:
- 正共振 (+R) 效应:当电子从与共轭体系连接的原子或取代基转移开时,就会发生正共振效应。例如苯胺中的+R效应

由于电子跨链传输,分子中的特定位点具有高电子浓度,这解释了这些位点的反应性。反映+R电子位移效应的基团包括卤素、-OH、-OR、-NH 2 、-NHR、-NR 2 、-NHCOR、OCOR等。
- 负共振(-R)效应:当电子向与共轭体系相连的原子或取代基转移时,就会产生负共振效应。例如,硝基苯中的-R效应

一些表现出-R电子置换效应的基团是-COOH、-CHO、-CN等。
超共轭
Hyperconjugation is the delocalization of electrons caused by the overlap of a p-orbital and a sigma (σ) bond (α C-H).
只有当 σ 键和空的 p 轨道正确定向时,才会发生超共轭。 Sigma 键电子与非共享的 p 轨道或连接的不饱和系统形成部分共轭。这是一种具有稳定作用的长期影响。
- 考虑乙基阳离子 CH 3 CH 2 。带正电的碳原子具有六个电子,杂化 sp 2 ,并具有未填充的 p 轨道。附近的一个甲基基团的 C-H 键与空的 p 轨道平面对齐。 σ 键(这个 CH 键)的电子离域到空的 p 轨道中,从而稳定了阳离子。
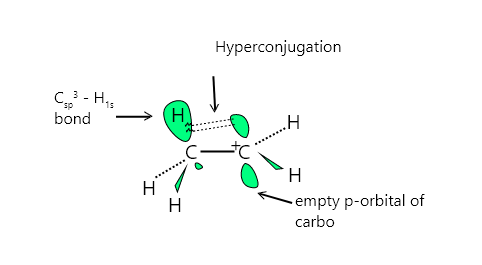
- 由于重叠,正电荷被周围 σ 键的电子密度扩散,从而稳定了阳离子。超共轭可以描述为,
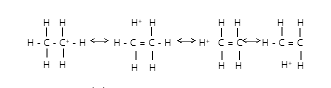
- 与带正电荷的碳原子连接的烷基越多,超共轭连接越强,阳离子越稳定。结果,以下阳离子的相对稳定性随着顺序的增加而降低。
(CH 3 ) 3 C + > (CH 3 ) 2 CH > CH 3 CH 2 > CH 3
- 这是因为叔丁基阳离子有九个超共轭结构,异丙基阳离子有六个,乙基阳离子有三个。因为 C + H 3中的空 p 轨道垂直于 CH 键所在的平面,所以重叠是不可想象的。结果,C + H 3缺乏超共轭稳定性。
- 通过超共轭的电子离域在烯烃和芳烃化合物(如烷基芳烃)中也是可行的。例如,丙烯中的超共轭如下图所示。

示例问题
问题 1:解释术语归纳效应?
回答:
Polarization of sigma bond caused by the polarization of adjacent sigma bond is called Inductive effect.
The inductive effect is related to the ability of substituents to either withdraw or donate electron density to attached carbon atom and hence classified as,
- Electron withdrawing group
- Electron donating group
问题2:在C 4 H 9 - Br 的哪个C - C 键中,预计感应效应最小?
回答:
We know that magnitude of inductive effect decreases as the number of intervening bonds increases.
So, consider, C4H3 – C3H2 – C2H2 – C1H2 – Br
The inductive effect is least in C3 – C4 bond.
问题3:写一个关于共振的笔记,也画出共振和共振混合的结构?
回答:
Resonance refers to the phenomena in which compounds exist in a state that is a mixture of two or more electronic structures, each of which appears equally capable of expressing most of the attributes of the compound but none of which describes all of the qualities. It is also called as mesomerism.
- A compound with delocalized electron is said to have resonance
- The actual structure which is composite of two structure is called resonance hybrid and is drawn by using dotted lines to show that electrons are delocalized
- Resonance forms are shown using double headed arrow between them
问题4:画出C 6 H 5 OH 的所有可能的共振结构?
回答:
The given compound, C6H5OH, is phenol. The possible resonance structures are,

问题 5:区分亲电试剂和亲核试剂
回答:
Electrophiles NucleophilesElectrophiles are electron-deficient species. Nucleophiles are electron-rich species. They are attracted towards negative charge. They are attracted towards positive charge. They attact electron-rich centre of the substrate. They attack electron-deficient centre of the substrate. These are Lewis Acid. These are Lewis Base. THese are also called electron-pain acceptors. These are also called electron-pair donor. These are cations or molecules having electron deficient atoms. These are anions or molecules containing atoms with atleast one lone pair of electrons.
问题 6:简要描述亲核试剂和碱基之间的区别。
回答:
A nucleophile is the term used when an electron-pair is donated to a species other than H*. for e.g. when electron pair is donated to the carbocation.
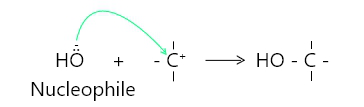
If an electron-pair is donated H+, it is termed as a base.

NH4+, Na+, K+, etc. are even though positively charged species but are not electrophiles as they do not have empty orbitals.