芳烃
在化学中,我们了解了烷烃、烯烃和炔烃。这些都是线性的。除了这些碳氢化合物,我们还有环状化合物。芳香烃是烃及其烷基、烯基和炔基衍生物,它们的分子中包含一个或多个稠合或分离的苯环。苯环是极度不饱和的,苯环的不饱和性在大多数芳香族化合物反应中都保留下来。具有苯环的芳香烃称为苯环,而没有苯环的称为非苯环。让我们看看什么是环状化合物,然后再了解芳香化合物。
什么是环状化合物?
A compound in which the atoms are linked together to form a ring or loop-like structure is termed a cyclic compound. They can be fully saturated or unsaturated i.e. they may contain only sigma bonds or both sigma and pi bonds.
环状化合物还有两种类型:
- 碳环:所有原子都是碳的化合物
- 杂环化合物:同时具有碳原子和非碳原子的化合物。
环状化合物的例子是环丙烷、环丁烷等。

环丙烷和环丁烷
既然我们已经了解了环状化合物,我们将研究芳香族化合物。
芳香族化合物
Cyclic Compounds that have planar rings with completely delocalized pi electrons are called aromatic compounds. They are also known as arenes. Aromatic compounds have a unique aroma and are hence termed aromatic compounds.
e.g.: Phenol, naphthalene, etc.

苯酚
在上图中,我们可以看到环具有完全离域的 pi 电子。根据芳香族化合物是否含有苯环,可分为苯类和非苯类。
芳香族化合物的性质是:
- 它们本质上是非极性的
- 由于它们的非极性性质,它们在水中不混溶
- 由于碳氢比高,它们燃烧时会发出灰黄色的火焰
- 它们用作非极性化合物的溶剂,通常不活泼。
- 它们经历亲电取代和亲核芳族取代反应。
仅通过查看其结构很难识别芳香族化合物。因此,我们利用了Huckel 规则。因此,芳香族化合物的定义可以扩展为符合 Huckel 规则的化合物称为芳香族化合物,其性质称为芳香性。
哈克尔法则
根据 Huckel 规则,芳香族化合物可能具有以下性质或满足以下条件:
- 平面性:化合物必须是平面的,即它不应该有任何碳-碳单键。化合物中的所有碳原子必须是 sp^2 杂化的。
- 离域 pi 电子:化合物必须在环上方和下方都有完全离域的 π 电子云。
- 4n+2 pi电子:这是 Huckel 规则最重要的条件。根据它,芳香族化合物必须有 4n+2 π 电子,其中 n 是整数,n = 0, 1, 2, 3, 4,…。
让我们看一个例子来验证 Huckel 规则。考虑如下所示的化合物,即苯
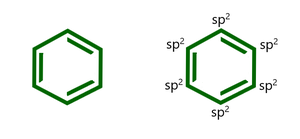
上述化合物本质上是平面的,因为所有碳原子都是 sp2 杂化的。该化合物还具有完全离域的 pi 电子。现在,没有。苯中 π 电子的数量为 6,当 n = 1 时等于 4(1)+2。因此它满足 Huckel 规则的所有条件,因此是芳香族化合物。
Anti Aromatic Compounds
Apart from aromatic compounds, there are antiaromatic compounds also. Anti aromatic compounds follow the first two conditions of Huckel’s rule but differ in the third one. They have (4n\π) electrons instead of (4n+2) \ π electrons. These compounds are very reactive in nature and highly unstable.
e.g.: Pentalene with 8πe−.

芳香族化合物的用途是:
- 人体内存在的核酸和氨基酸本质上是芳香的
- 植物中存在的叶绿素也是一种芳香化合物,是植物生存和制造食物所必需的。
- 萘是一种芳香化合物,用于制作樟脑丸以保护衣服。
- 三硝基甲苯用于制造炸药
- 它们被用来制造药物和染料
- 它们广泛用于塑料和石化工业。
示例问题
问题 1:定义芳香族化合物。
回答:
Cyclic Compounds that have planar rings with completely delocalized pi electrons are called aromatic compounds. They are also known as arenes. Example: Benzene, Naphthalene
问题 2:定义抗芳香族化合物。
回答:
Compounds that are planar in nature and have completely delocalized pi electrons but have 4n\pi electrons are called anti aromatic compounds.
问题 3:陈述芳香族化合物和反芳香族化合物之间的两个区别。
回答:
The difference between aromatic and anti aromatic compounds are as follows: Aromatic Anti AromaticThey have 4n+2 pi electrons They have 4n pi electrons They are very stable and unreactive They are highly reactive in nature
问题 4:State Huckel 规则。
回答:
According to Huckel’s rule, the aromatic compounds may possess the following properties or satisfy the following criteria:
- Planarity: The compound must be planar i.e. it should not have any Carbon-Carbon single bonds. All the Carbon atoms in the compounds must be sp^2 hybridized.
- Delocalized pi electrons: The compound must have a completely delocalized \pi electron cloud both above and below the ring.
- 4n+2 \pi electrons: This is the most important condition of the Huckel’s rule. According to it, an aromatic compound must have 4n+2 \pi electrons where n is an integer and n = 0, 1, 2, 3, 4,………
问题 5:陈述芳香族化合物的两种用途。
回答:
Following are the two uses of aromatic compounds are:
- Naphthalene which is an aromatic compound is used to make mothballs to protect clothes.
- Tri Nitro Toluene, an aromatic compound, is used to make explosives.
问题 6:说出芳香族化合物的任意两个性质。
回答:
Following are the two properties of aromatic compounds are:
- They are immiscible in water due to their non polar nature.
- They burn with a sooty yellow flame due to high Carbon-Hydrogen ratio.
问题 7:验证给定的化合物是否是芳香族的。

回答:
The compound shown in above image is planar in nature as all the Carbon atoms are sp2 hybridized but it has 4 pi electrons which violates Huckel’s rule. Hence it is not aromatic but anti aromatic.