热容量
物质的热特性导致物质传导热量或在存在热量的情况下决定物质的性质。因此,当热量通过物体时,它会表现出热特性。不同的材料或事物根据其热特性在热的影响下表现不同。或者,这些品质决定了物质在受热变化时如何反应。
热特性分为四类:热容量、热膨胀、热导率和热应力。
让我们看一些示例,将容器装满一半的水,然后将其放在炉子上加热。泡沫开始上升。随着温度的升高,水粒子的速度增加,并且随着水开始沸腾而变得湍流。
- 第一阶段是将指定量的水加热到例如 20 摄氏度的温度,并记录所需的时间。使用相同的热源,将等量水的温度升高40摄氏度,并记录时间。据观察,它花费了将近两倍的时间,因此,提高相同数量的水的温度所需的热量增加了一倍。

图1
- 现在在第二步中取双倍量的水并使用相同的加热系统将其加热以将温度升高20°C,据观察所需的时间将是第一步的两倍。
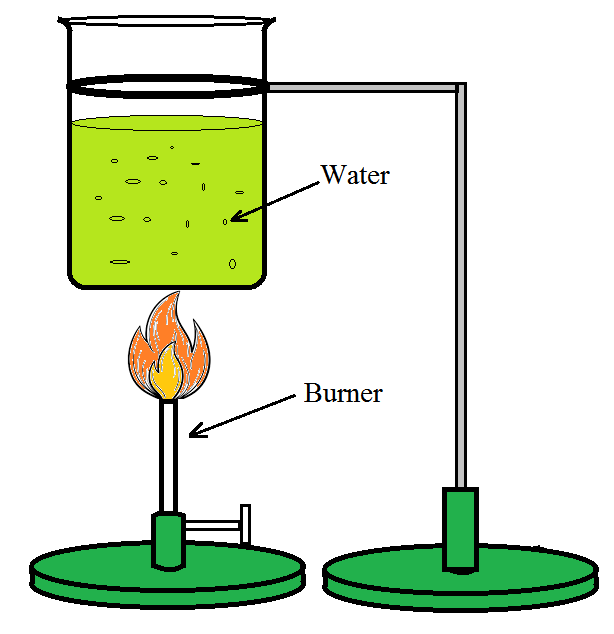
图 2
- 在第三阶段,加热等量的油,代替水,将温度升高20摄氏度。记录时间。观察到所需的时间更短,因此,所需的热量少于相同体积的水在相同的温升下所需的热量。

图 3
什么是热容量?
The change in temperature of a substance, when a given quantity of heat is absorbed or rejected by it, is characterized by a quantity called the heat capacity of that substance. It is denoted by S.
它表示为,
S= ΔQ / ΔT
其中 ΔQ 是提供给物质以将其温度从T变为T + ΔT的热量
比热容
当对相同质量的不同物质施加相同的热量时,产生的温度变化是不一样的。它表明每一种物质为了改变其单位质量的温度一个单位而吸收或释放的热量具有不同的值。物质的比热容就是这个量的量度。它由字母 s 表示。
如果 S 是质量为m的物质在经历温度变化ΔT时吸收或释放的热量,即ΔQ ,则该物质的比热容由下式给出
s = S/m = (1/m) (ΔQ / ΔT)
Specific heat capacity is defined as the number of heat changes i.e. heat absorbed or rejected by a substance per unit mass in order to change its temperature by one unit.
比热容是物质的特性,它决定了一定量物质的温度变化,即吸收或释放的热量。
给定物质在温度变化期间没有发生相变。它取决于物质的性质和温度。
J kg –1 K –1是比热容的 SI 单位。
摩尔比热容
如果物质的量以摩尔为单位而不是以千克为单位的质量 m,则物质每摩尔 μ 的热容可计算如下:
C = S/μ = (1/μ)(ΔQ/ΔT)
其中 C 表示物质的摩尔比热容。它还取决于物质的性质和温度。
SI 单位为Jmol –1 K –1 。
The molar specific heat capacity at a fixed pressure, denoted by Cp, is the molar specific heat capacity at fixed pressure when the gas is held at fixed pressure during the heat transfer. If the volume of the gas is kept fixed during the heat transfer, the corresponding molar specific heat capacity is known as molar specific heat capacity at constant volume and is indicated by Cv.
与其他物质相比,水在大气压下具有最高的比热容。这就是为什么水被用作汽车散热器中的冷却剂和热水袋中的加热器。在夏季,由于比热容大,海水比陆地升温慢得多,因此,来自海面的风会产生降温作用。
让我们还讨论其他热特性:
导热系数:
并非所有材料都能够通过它们的身体传递热量。指挥是那些能做到的人。由于它们的导热性,这些材料允许热量通过它们。有导电率高的导体,这意味着它们比导热率低的导体传导更多的热量。绝缘体是不以任何方式导热的材料。
热应力:
金属具有传输或传递热量的能力,这是它们的品质之一。这种转移会导致物理变化,例如温度升高时膨胀和温度下降时收缩。这发生在三个维度中的每一个维度中。当温度变化时,金属结构构件的热膨胀会引起热应力。结构构件的热变形是由温度变化引起的。
Thermal stress has the ability to harm a thing in some cases, hence it could be destructive in nature. For example, large truck tires develop cracks. This is because high-speed driving causes heat to be produced by friction between the road’s surface and the tires. The tires crack as a result of the resulting heat stress.
温度和热量:
Temperature is a measure of hotness or coolness that is relative. A hot utensil has a high temperature, whereas an ice cube has a low temperature. A hotter object is one that has a higher temperature than another one. Hot and cold, like tall and short, are relative concepts.
在炎热的夏日,桌上的一杯冰水慢慢变热,而同一张桌子上的一杯热咖啡却变凉了。当机体的温度(在这种情况下为冷水或热咖啡)与周围介质的温度不同时,系统和周围介质之间会继续发生热传递,直到机体和周围介质达到相同的温度.
Heat flows from the environment to a glass tumbler of chilled water, but it flows from the cup of hot coffee to the environment in the case of hot coffee. As a result, it is defined heat as the form of energy exchanged through temperature differential between two (or more) systems or between a system and its surroundings.
传递热能的国际单位制是焦耳(J),而温度的国际制单位是开尔文,用K表示,常用的温度单位是摄氏度,用℃表示。物体受热时会发生许多变化。它的温度可以改变,它可以生长,它可以改变状态。
状态变化:
固体、液体和气体是物质的三种基本状态。状态变化是从这些状态之一到另一种状态的移动。固体到液体和液体到气体是两种常见的状态转变(反之亦然)。当物质与其周围环境进行热交换时,会发生某些变化。
熔化是将固体转变为液体,而熔化是将液体转变为固体。在从固体到液体的转变过程中,物质的固相和液相以热平衡共存。物质的熔点是其固态和液态相互处于热平衡的温度。这是物质的属性。这也取决于压力的大小。物质的正常熔点是其在标准大气压下的熔点。
从液体到蒸汽(或气体)的转变称为汽化。发现温度保持恒定,直到全部液体变成蒸气。也就是说,在从液态到气态的整个转变过程中,物质的液态和气态以热平衡共存。物质的液态和气态共存的温度称为沸点。
潜热
The heat or energy absorbed or released during a phase shift of a substance is known as latent heat.
它可以是从气体到液体或从液体到固体。焓是与潜热相关的热属性。然而,关于潜热要记住的一个关键因素是物质的温度不会改变。就该过程而言,潜热是克服将物质中的分子和原子保持在一起的吸引力所需的努力。

在沸点,液体会改变其状态。当您向沸水添加额外的热量时,它会在不增加温度的情况下蒸发。状态变化期间所需的热量由转变热和物质变化状态的质量决定。因此,如果一种物质的质量 m 从一种状态变为另一种状态,则所需热量由下式给出
Q = m L
要么
L =Q/m
L 代表潜热,它是物质的一种特性。 J kg –1是它的 SI 单位。 L 的值同样受压力影响。它的值通常用普通大气压来表示。熔化潜热 (L f ) 用于描述固-液状态变化,而汽化潜热 (L v ) 用于描述液-气状态变化。
示例问题
问题 1:将 95.24 C 的 88.3 g 金属样品加入 35.10 g 最初温度为 17.27 °C 的水中。水和金属的最终温度均为 29.20 °C。水的比热为 4.184 J/(g°C)。计算金属的比热。
解决方案:
Given,
Mass of metal is 88.3 g
The initial temperature of the metal is 95.24 °C.
Mass of water: 35.10 g.
The initial temperature of the water is 17.27 °C.
The final temperature of the water and the metal is 29.20 °C.
The specific heat of water is 4.184 J/(g°C).
Therefore, the expression where the energy from the hotter metal transfers to the cooler water is
−moCoΔTo=mwCwΔTw
Where
mo= mass of a metal object
ΔTo = temperature change of metal object
Co= specific heat capacity of metal object
mw= mass of water
ΔTw= temperature change of water
Cw= specific heat capacity of water
Rearrange the above expression,
Co=(mwCwΔTw)/(moΔTo)
Substitute the values in the above expression,
Co=[35.10 4.184(29.20−17.27)]/[88.3(29.20-95.24)]
Co=0.301 J/g°C
问题2:定义热容量并写出它的表达式。
解决方案:
The change in temperature of a substance, when a given quantity of heat is absorbed or rejected by it, is characterized by a quantity called the heat capacity of that substance. It is denoted by S.
It is expressed as,
S= ΔQ/ ΔT
where ΔQ is the amount of heat supplied to the substance to change its temperature from T to T + ΔT
问题3:水的比热为4.18 J/(g°C)。计算水的摩尔热容。用三位有效数字表达你的答案并包括适当的单位。
解决方案:
The specific heat of water is 4.18 J/(g°C).
The expression to convert gram into mole is
4.18 J/gC x (18.0 g / mole) = 75.24 J/mole C
Hence, the molar heat capacity of water is 75.24 J/mole C
问题4:定义比热容并写出其表达式。
解决方案:
When the same quantity of heat is applied to the same mass of different substances, the resulting temperature changes are not the same. It indicates that each substance has a distinct value for the quantity of heat absorbed or rejected to change the temperature of its unit mass by one unit. This quantity is referred to as the specific heat capacity of the substance. It is denoted by s.
If ΔQ stands for the amount of heat absorbed or rejected by a substance of mass m when it undergoes a temperature change ΔT, then the specific heat capacity, of that substance is given by
s = S/m = (1/m)(ΔQ/ ΔT)
The SI unit for specific heat capacity is Jkg–1 K–1 .
问题 5:定义摩尔比热容并写出其表达式。
解决方案:
If the amount of substance is provided in moles rather than mass m in kilograms then the heat capacity per mole μ of a substance can be calculated as follows:
C = S/μ = (1/μ)(ΔQ/ ΔT)
where C denotes the substance’s molar specific heat capacity. It also depends on the nature and temperature of the substance and its SI unit is Jmol–1 K–1.
The molar specific heat capacity at fixed pressure, denoted by Cp, is the molar specific heat capacity at fixed pressure when the gas is held at fixed pressure during the heat transfer. If the volume of the gas is kept fixed during the heat transfer, the corresponding molar specific heat capacity is known as molar specific heat capacity at constant volume and is indicated by Cv.
问题 6:将 30.5 g 93.0 °C 的合金样品放入热容量为 9.2 J/K 的隔热咖啡杯中的 50.0 g 22.0 °C 水中。如果系统的最终温度为 31.1 °C,合金的比热容是多少?
解决方案:
Heat absorbed = heat lost
then the specific heat capacity, of that substance is given by
s = (1/m)(ΔQ/ ΔT)
Rearrange the above expression,
ΔQ=smΔT
ΔQalloy = ΔQwater+ΔQcup
The temperature of the water is equal to the temperature of the cup = 22.0 °C.
The temperature of the alloy is 93.0 °C.
The final temperature is 31.1 °C.
30.5×(93.0 – 31.1)s = 9.2×(31.1-22.0) + 50.0×4.2×(31.1-22.0)
1887.95s = 1994.72
s = 1.057 J/gK