分子间力与热相互作用
我们周围的一切都是具有某些物理和化学性质的物质。我们周围的物质存在于不同的状态。为了使物质以不同的状态发生,必须在该物质的原子之间存在某种力才能将原子保持在一起。让我们了解导致物质存在的这些力量。力可以理解为某种固定物或将它们结合在一起的紧固件。
在原子的情况下,需要某种力来将原子粒子电子、质子和中子保持在原子内部并将它们保持在一起以保持原子的稳定性。由于存在不同的力以提供稳定性,电子在原子核周围的圆形轨道中移动。
分子间作用力
Intermolecular forces can be defined as the attraction or repulsion forces that are applied to atoms and molecules when they interact with each other whether at the time of phase change or in chemical reactions. Electrostatic forces which are present between ions having different charges are not considered intermolecular forces.
当这些分子间力具有吸引力时,它们也被称为范德华力。为了纪念伟大的科学家约翰内斯·范德华,分子间作用力被称为范德华力,因为他解释了真实气体行为的概念,该行为偏离了在标准温度和压力下表现出的理想行为。
随着分子间吸引力的增加,沸点升高。相反,可以通过比较它们的沸腾温度来评估各种物质的分子间作用力。这是因为沸点处的热量导致分子间连接破裂,将液体转化为蒸汽。同样,随着分子间相互作用变得更加强烈,熔点也会升高。
分子间作用力的类型
分子间作用力主要有以下五种类型:
分散部队或伦敦部队
Dispersion Forces or London Forces can be defined as the attraction forces which are being applied between two temporary dipoles. These forces distort the electron cloud density of atoms. These forces are attractive in nature and these are named after a German physicist Fritz London and that’s why they are also known as London Forces.
让我们用一个例子来理解这些力,所以取两个原子 X 和 Y,它们彼此非常接近,但没有接触,所以如果在任何原子中受到干扰,那么电荷分布在任何时间点,然后由于伦敦力的影响其他原子的电荷云也会受到干扰。
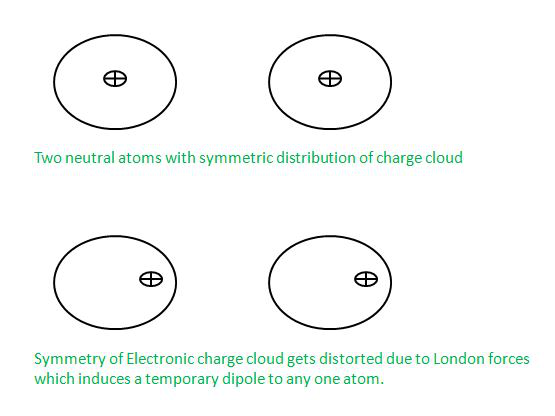
偶极-偶极力
Dipole-Dipole Forces can be defined as the forces which act between the molecules having permanent dipole. The dipole can be understood as a pair of opposite charges on a molecule or on a bond.
让我们通过一个例子来理解作用在 HCl 分子之间的偶极-偶极力,因为 HCl 分子具有永久偶极子,因此在 Cl 侧,电子电荷云密度大于氢侧。

偶极诱导偶极力
Dipole–Induced Dipole Forces can be defined as the attraction forces which are being applied between two molecules in which one has a permanent dipole and the other does not have a permanent dipole. Hence the molecule with permanent dipole induces the dipole to the other molecule which is electrically neutral and hence distorts the electronic charge cloud.

离子偶极相互作用
这是极性分子和离子(阳离子或阴离子)之间的吸引力。当 NaCl 溶解在水中时,极性水分子会被 Na +和 Cl -离子吸引(这一过程称为离子水合)。这种接触的强度取决于极性分子的偶极矩和大小,以及离子的电荷和大小。因为阳离子的电荷密度高于同电荷的阴离子,所以这种接触通常与阳离子更强。此外,由于 CCl 4是非极性的,它不能与阳离子 Na +和 Cl -相互作用。结果,NaCl不溶于CCl 4 。
因为任何离子的电荷远大于偶极矩的电荷,所以离子-偶极吸引力强于偶极-偶极相互作用。
离子诱导偶极相互作用
非极性分子附近存在离子会导致其极化,从而使其成为诱导偶极子。离子诱导的偶极相互作用是它们之间的相互作用。这些相互作用的强度由离子的电荷和非极性分子极化的情况决定。阴离子通过排斥使分子极化,而阳离子通过电子云的吸引使分子极化。
例如,血红蛋白存在于红细胞 (RBC) 中。它的核心有一个 Fe2+ 离子,它通过离子诱导的偶极力吸引 O2 离子。
热能
系统内部包含的对其温度负责的能量称为热能。热能的传递称为热量。热力学是一门物理学学科,研究热量如何跨系统传递以及在此过程中如何完成工作(参见热力学第一定律)。
Thermal Energy can be defined as the energy which arises due to the motion of atoms and molecules of any system. The motion of particles of a system is known as thermal motion which gives rise to thermal energy and this thermal energy varies directly with the temperature.
由于运动而产生的单个粒子的能量称为动能,但所有粒子的平均能量给出了热能的量度。
热能和分子间力之间的差异
Thermal Energy | Intermolecular Forces |
| It is due to the motion of particles. | It is due to the dipole of one or both molecules. |
| It has the tendency to keep particles apart. | It has the tendency to hold particles together. |
| A large amount of thermal energy means matter cannot exist in the solid phase. | A Large number of intermolecular forces means matter can exist as solids. |
| Thermal energy is directly proportional to temperature. | Intermolecular forces do not have much effect on temperature. |
| In the gaseous phase, thermal energy is high. | In the gaseous phase, intermolecular forces are weak. |
| In solid-phase, there is a negligible amount of motion of particles due to the least thermal energy. | In solid-phase, there is a large amount of intermolecular force between particles so there is no motion. |
| Gases can be liquefied to reduce thermal energy. | Gases can’t liquefy even if intermolecular force is maximum |
| The volume of the matter is more if thermal energy is high. | Volume of the matter is less if intermolecular forces are maximum |
| Three states of matter occur due to the balance of thermal energy. | Three states of matter occur due to the balance of intermolecular forces. |
示例问题
问题一:为什么液体和固体都难以压缩?
回答:
Molecules of the same or different elements exert repulsive forces also on each other. When two molecules come very close to each other, the force of repulsion between the electron clouds of the molecules and the force between the nuclei of two molecules comes into existence. The strength of these repulsion forces rises very rapidly as the distance between the molecules decreases and vice versa. This is the reason that liquids and solids are hard to compress.
问题2:什么是氢键?
回答:
Hydrogen bonding can be understood as a special powerful type of dipole-dipole interaction. Hydrogen bond formation is shown by only a few elements so it is kept separately from other intermolecular forces. Molecules which are strongly polar shows the tendency to form hydrogen bonding so molecules of Nitrogen, Oxygen and Fluorine are able to make hydrogen bonds.
问题3:伦敦力的相互作用能如何取决于两个粒子之间的距离?
回答:
The interaction energy of London forces varies inversely to the sixth power of the distance between two particles.
Interaction Energy ∝ 1/x6
where x is the distance between two particles
问题 4:氢键是否仅限于氮、氧和氟分子?
回答:
Yes, it is true that hydrogen bonding is considered limited to Nitrogen, Oxygen and Fluorine, but there are some special cases where hydrogen bonding can be seen in Chlorine also. Hydrogen bonds have a large amount of energy somewhat between 10 to 100 kJ mol-1, so hydrogen bonds are very powerful hence these helps in the determination of the structure and properties of many compounds.
问题5:偶极-偶极力的相互作用能如何取决于两个粒子之间的距离?
回答:
Dipole-dipole forces are between polar molecules which are of two types one is Stationary and the other is Rotating. The interaction energy of dipole-dipole forces varies inversely to the third power of the distance between two particles in the case of stationary polar molecules.
Interaction Energy ∝ 1/x3
where x is the distance between two particles.
The interaction energy of dipole-dipole forces varies inversely to the sixth power of the distance between two particles in the case of rotating polar molecules.
Interaction Energy ∝ 1/x6
where x is the distance between two particles.