摩尔浓度公式
描述特定溶液中溶质浓度的最方便的形式是摩尔浓度。每升溶液中溶解的溶质的摩尔数称为摩尔浓度。因此,M = 摩尔每升。溶液的摩尔量,即摩尔浓度,将由所有摩尔计算确定。

酸和碱之间的摩尔比始终是平衡化学方程式的结果。结果,碱中的摩尔数增加。通过将摩尔数除以溶液的体积,我们可以得到摩尔浓度。所有摩尔值都用于计算摩尔浓度,即溶液中摩尔的体积。
公式
溶液的摩尔浓度计算为摩尔量与给定溶液体积的比值。其公式如下:
Molar Concentration = Amount in moles/Volume of the solution
示例问题
问题 1. 如果 16.35 mL AgNO 3溶液与 0.0003017 kg KIO 3 反应,求其浓度。
解决方案:
AgNO3 + KIO3 = AgIO3 + KNO3
Number of moles in KIO3 = Mass/Molar mass
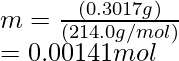
Hence, Number of moles in AgNO3 = 0.00141 mol
Now, Concentration of AgNO3 = Moles/Volume
Concentration of AgNO3 = (0.00141)/(0.01635)
= 0.08624 M
问题 2. 摩尔浓度公式是做什么用的?
解决方案:
The concentration formula in chemistry is utilized in a variety of branches of study. This formula is frequently used in advanced science courses to assist students comprehend the kinetics of chemical reactions. In fact, molarity may be used to evaluate the influence of thermal expansion in fluids. This formula can be used to compute the amounts of reacting substances or the quantity necessary for a reaction to produce a calculated output.
问题 3. 如果在 200 毫升溶液中,求 4 克苛性钠的摩尔浓度。
解决方案:
Mass = 4 g
Volume = 200 ml
Molar mass of caustic soda = 23 + 16 + 1 g
= 40 g
Molarity = Mass/Molar Mass
= 4g/40g
= 0.1 g
问题 4. 使用 15 克硫酸钠制成溶液。该溶液的体积为 125 毫升。找出提供的硫酸钠溶液的摩尔浓度。
解决方案:
Molar mass of sodium sulphate = 23 × 2 + 32 + 16 × 4 = 142 g
Number of moles in sodium sulphate = 15/142 = 0.106
Volume of the solution = V = 0.125l
Thus, molarity = n/V
= 0.106/0.125
= 0.85 M
问题 5.确定氢氧化钠与盐酸反应的摩尔浓度。
解决方案:
HCl + NaOH → NaCl + H2O
Number of moles in HCl = Number of moles in NaOH = 8.75 × 10-3 mol.
The amount of NaOH present in moles is
Volume of solution = 25 × 10-3dm3
M = 8.75 × 10−3/25.0 × 10-3
= 0.350 mol dm-3