吉布斯能量变化与平衡
能量可以有多种形式,包括物体运动产生的动能、物体位置产生的势能、由于温差从一个物体传递到另一个物体的热能、与阳光相关的辐射能、原电池产生的电能,储存在化学物质中的化学能,等等。所有这些不同类型的能量都可以从一种形式转变为另一种形式。
例如,当大坝水库中的水落下时,其势能转化为动能,如果将落下的水用于驱动涡轮机,则水的动能转化为电能。但是,如果水与大坝底部附近的岩石发生碰撞。物体的动能转化为热能。
结果,各种能量在数量上相互联系在一起。热力学是研究各种能量之间的这种定量关系。能量转移是物理和化学过程的结果。研究这些过程中的能量转变是热力学的主要焦点。
Thermodynamics is the branch of science that studies the many kinds of energy, their quantitative connections, and the energy changes that occur in physical and chemical processes.
吉布斯能源
JW吉布斯 是一位美国理论家。他引入了一个新的热力学函数,称为吉布斯能量,记为 G。热力学第二定律指出,对于所有自发过程,ΔS总= (ΔS系统+ ΔS环绕) 必须为正。为了评估过程的自发性,必须确定两个熵变化,ΔS System和 ΔS Surrounding 。因此,在不考虑环境的情况下,仅根据系统的热力学特征来描述自发性的标准更为直接,这个问题被Gibbs解决了。
Gibbs energy (G) is defined as,
G = H – TS
where:
- S is the entropy of the system,
- H is Enthalpy, and
- T is the Temperature.
G is also a state function because, H, T, and S are state functions.
吉布斯能量 (ΔG) 在系统的初始和最终状态上的变化,而不是在连接这两个状态的路径上。恒温恒压下吉布斯能量的变化定义为:
ΔG = ΔH – T ΔS
where:
- ΔS is the change in entropy of the system and
- ΔH is the change in Enthalpy.
吉布斯能量和自发性
总熵变可以写为,
ΔS总= ΔS系统+ ΔS周围= ΔS + ΔS surr
根据热力学第二定律,在恒定的温度和压力下,ΔS total > 0 表示该过程是自发的。如果ΔH是伴随反应的焓变,即系统的焓变,那么周围的焓变为(-ΔH)
所以,
ΔS surr = -ΔH / T
因此总熵由下式给出,
ΔS总= ΔS – ΔH / T
该等式表明,ΔS total仅根据系统的属性表示。
重新排列我们得到的方程,
-T ΔS总计= -T ΔS + ΔH
要么
-T ΔStotal = ΔH -T ΔS
结合上述两个方程,我们得到,
ΔG = -T ΔS总计
This equation indicates that ΔG and ΔStotal have opposite signs because T is always positive. Thus, for a spontaneous process carried out at a constant temperature and pressure ΔStotal > 0 and hence ΔG < 0.
Gibbs energy of a system decreases in a spontaneous change that takes place at constant temperature and pressure. On contrary, for a non-spontaneous reaction ΔStotal and hence ΔG > 0.
Gibbs energy of a system increases in a non-spontaneous change that takes place at constant temperature and pressure. The end of the spontaneous process is an equilibrium that corresponds to a minimum in G. Hence the change in Gibbs energy is:
- ΔG < 0, the process is spontaneous.
- ΔG > 0, the process is non-spontaneous.
- ΔG = 0, the process is at equilibrium.
影响自发性的因素
考虑方程,
ΔG = ΔH – T ΔS
ΔG值决定了物理或化学变化是否会自发发生。方程 ΔH 和 ΔS 仅对应于系统的值。该方程表明,两种元素会影响反应的自发性
- ΔH 是在恒定压力和温度下传递的热量,并且
- ΔS 是分子无序的上升或下降。
自发过程有利于焓降低(-ΔH)和熵增加(ΔS),另一方面,非自发反应有利于焓增加(+ΔH)和熵减少(-ΔS)。方程中的温度项是确定焓和熵对 ΔG 贡献的相对相关性的重要组成部分。如果方程中的 ΔH 和 ΔS 都为正或均为负,则 ΔG 的符号以及反应的自发性取决于温度。 ΔH ΔS ΔG Spontaneity of reaction Negative (exothermic) Positive Negative Reactions are spontaneous at all temperatures. Negative (exothermic) Negative Negative or Positive Reactions become spontaneous at low temperatures when |T. ΔS| < |ΔH|. Positive (endothermic) Positive Negative or Positive Reactions become spontaneous at low temperatures when T.ΔS < ΔH. Positive (endothermic) Negative Positive Reactions are non-spontaneous at all temperatures.
平衡温度
At equilibrium, i.e., ΔG = 0, the process is neither spontaneous nor non-spontaneous because it is balanced between spontaneous and non-spontaneous behavior. (+ΔH)
So,
ΔG = ΔH – T ΔS = 0
Hence,
ΔH = TΔS or T = ΔH / ΔS
T is the temperature at which the transition from spontaneous to non-spontaneous behavior happens. T is calculated on the assumption that ΔH and ΔS are temperature independent. In reality, ΔH and ΔS change with temperature. However, for modest temperature changes, the variance in them will not add considerable mistakes.
ΔG 和平衡常数
化学反应中的所有物质(反应物和产物)可能不是它们的正常形式。由于这种联系,反应吉布斯能量的变化与标准吉布斯能量的变化有关。
ΔG = ΔG° + RT ln Q
where:
- ΔG° is the standard Gibbs energy change (change in Gibbs energy when all the substances are in their standard state).
- Q is the reaction quotient.
反应商的表达与平衡常数的表达类似,但它们之间只有一个区别,即平衡常数包括产物和反应物的平衡浓度或分压。而 Q 以反应物起始浓度分压和产物最终浓度或压力表示。
例如,请考虑以下示例:
aA +bB ⇢ cC + dD
对于上述反应,反应商由下式给出
![]()
要么
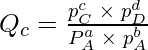
当浓度或分压值不是平衡值时。当反应达到平衡时,浓度和分压达到平衡值,此时Q = K。平衡时,ΔG = 0,Q = K,则标准吉布斯能量方程变为,
0 = ΔG° + RT ln K
因此,
ΔG° = -RT ln K = -2.303RT log10K
该方程给出了反应的标准吉布斯能量变化与其平衡常数之间的关系。
示例问题
问题 1:确定对于给定的 ΔH 和 ΔS 值,反应是自发的还是非自发的。另外,说明它们是放热的还是吸热的。
- ΔH = – 40 kJ 和 ΔS = +135 JK -1在 300K
- ΔH = – 60 kJ 和 ΔS = -160 JK -1在 400K
解决方案:
- ΔG = ΔH – T ΔS
ΔH = – 40 kJ, ΔS = +135 J K-1 = 0.135 kJ K-1 and T = 300K
ΔG = -40 (kJ) – 0.135(kJ K-1) × 300(K)
= 80.5 kJ
Because ΔG is negative, the reaction is spontaneous. The negative ΔH value indicates that the reaction is exothermic.
- ΔG = ΔH – T ΔS
ΔH = – 60 kJ, ΔS = – 160 J K-1 = – 0.160 kJ K-1 and T = 400K
ΔG = -60 (kJ) – 0.160(kJ K-1) × 400(K)
= -60 kJ + 64 kJ = 4kJ
The reaction is non-spontaneous because ΔG is positive and exothermic as ΔH is negative.
问题 2:对于某个反应 ΔH = -25kJ 和 ΔS = -40J K -1 。在什么温度下它会从自发变为非自发。
解决方案;
T = ΔH / ΔS
ΔH = – 25 kJ, ΔS = -40 J K-1 = 0.04 kJ K-1
Hence, T = -25(kJ) / -0.04 kJ K-1 = 625K
Because both ΔH and ΔS are negative, the reaction will occur spontaneously at lower temperatures. As a result, the reaction will be spontaneous below 625K and non-spontaneous beyond 625K.
At 625K, the transition from spontaneous to non-spontaneous occurs.
问题 3:确定 ΔS total并确定以下反应是否在 298K 时是自发的。
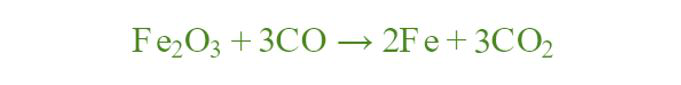
ΔH° = -24.8 kJ,ΔS° = 15 JK -1
解决方案:
The heat evolved in the reaction is 24.8 kJ. The same quantity of heat is absorbed by the surroundings.
Hence, Entropy change of the surrounding will be,
ΔSsurr = ΔH° / T
= – [(-24.8 (kJ)) / 298 (K)]
= + 83.2 J K-1
ΔStotal = ΔSSystem + ΔSSurr
ΔSSys = ΔS° = 15 J K-1
= 15(J K-1) + 83.2 (J K-1)
= 98.2 J K-1
As ΔStotal is positive, the reaction is spontaneous at 298 K.
问题4:判断是否反应,
N 2 O 4 (克)⟶2NO 2 (克)
根据以下数据,在 298 K 时是自发的。
Δ f H° (N 2 O 4 ) = 9.16 kJ mol -1 , Δ f H° (NO 2 ) = 33.2 kJ mol -1
解决方案:
ΔH=∑ΔfH°(products)−∑ΔfH°(reactants
= 2 × ΔfH° (NO2) – ΔfH° (N2O4)
= 2(mol) × 33.2(kJ mol-1) -1(mol) × 9.16(kJ mol-1)
= +57.24 kJ
ΔG° = ΔH° – TΔS°
57.24(kJ) – 298(K) × 175.8 × 10-3 (kJ K-1)
= +4.85 kJ.
Because ΔG° is positive, the reaction is non-spontaneous at 298 K.
The temperature at which the reaction changes from spontaneous to non-spontaneous is given by,
T = ΔH° / ΔS°
= 57.24(kJ) / 0.1758(kJ K-1)
= 325.6 K
Because ΔH° and ΔS° are both positive, the reaction will be spontaneous at high temperature.
The reaction will be spontaneous above 325.6 K.
问题 5:确定反应的Kp ,
2SO 2 (g) + O 2 (g) ⟶ 2SO 3 ( g)
在 298 K 时为 7.1 × 10 24。计算反应的 ΔG° (R = 8.314 JK -1 mol -1 )。
解决方案:
ΔG° = -2.303RT log10 Kp
Kp = 7.1 × 1024
R = 8.314 JK-1 mol-1
T = 298K
Hence,
ΔG° = -2.303 × 8.314 × 10-3 (kJ K-1 mol-1) × log10 (7.1 × 1024)
= -141.8 kJ mol-1
问题陈述 6:计算 513 K 反应的 K p ,
2NOCl (g) ⟶ 2NO(g) + Cl 2 (g)
ΔG° = 17.38 kJ mol -1 。
解决方案:
ΔG° = -2.303 RT log10 Kp
ΔG° = 17.38 kJ mol-1
R = 8.314 J K-1 mol-1
T = 513K
Hence,
log10 kp = – ΔG° / 2.303 RT
= – (17380(J mol-1) / 2.303 × 8.314 ( J K-1 mol-1) × 513(K))
= -1.769
Hence, Kp = antilog(-1.769)
= 0.017