电流的化学效应
建议不要用湿手接触任何电器。用湿手操作电器是很危险的,因为它会对我们造成伤害。良好的电导体是允许电流流过它们的材料。另一方面,不容易让电流流过它们的材料是不良的电导体。已发现铜和铝等金属可以带电,而橡胶、塑料和木材则不能。

用于检查蒸馏水和不纯水中导电情况的测试仪。
当测试仪两端之间的液体允许电流通过时,测试仪的电路就完成了。当电流流过电路时,灯泡会亮起。测试仪的电路不完整,当液体不允许电流流动时,灯泡不发光。
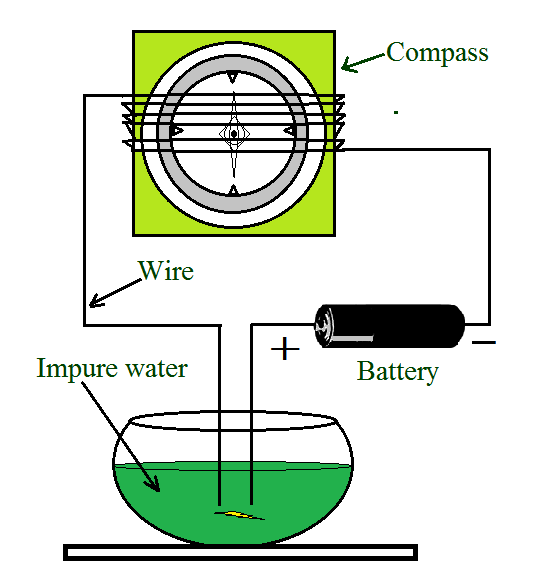
另一个测试员
尽管材料可以带电,但它可能无法像金属一样带电。测试仪的电路可能是完整的,事实上流过它的电流可能太弱而无法点亮灯泡。在这种情况下,电流的影响被认为是创建不同类型的测试仪。不管电流是否小,都可以观察到磁针的偏转。利用电流的磁效应制成测试仪。
电流的化学作用
当它流过导电溶液时,它总是会产生一些效果。当电流通过导电溶液时会发生化学反应。结果,电极上可能会形成气泡,电极上可能会检测到金属沉积物,并且溶液的颜色可能会发生变化。所用溶液和电极的类型将决定反应及其效果。
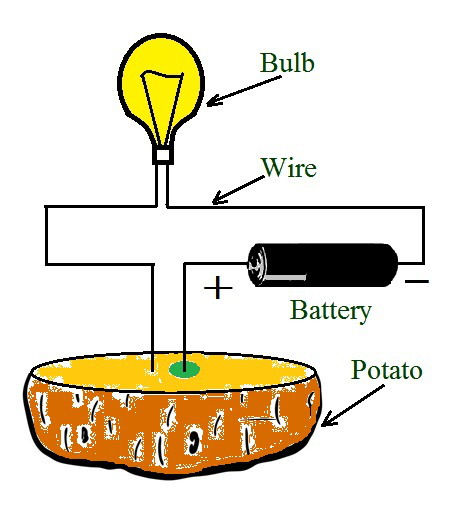
测试马铃薯
让我们考虑一个实验,看看一些水果和蔬菜是否同样可以导电。将马铃薯切成两半,将测试仪的两个铜端子或电线插入马铃薯的一半。半小时后,观察到一根电线周围的马铃薯上有一个蓝绿色的标记,而另一根电线周围没有这样的斑点。始终是连接到正极端子的电线周围有一个蓝绿色的斑点。
这个实验旨在测试马铃薯是否导电。对该实验进行了测试,以便查看马铃薯中产生的化学效应电流。
测试仪:测试仪是一种电气设备,用于确定是否存在电流。它通常是带有 LED 或灯泡的导体,以显示电路中有电流。
电镀:经常看到女性佩戴看起来是由黄金制成的珠宝。然而,金色的覆盖物会随着时间的推移而褪色,露出下面的银或其他金属。在这种情况下,金属具有另一种金属的覆盖层。
Electroplating is the process of depositing a layer of any desired metal on another material using electricity. It’s one of the most frequent uses of electric current’s chemical effects.
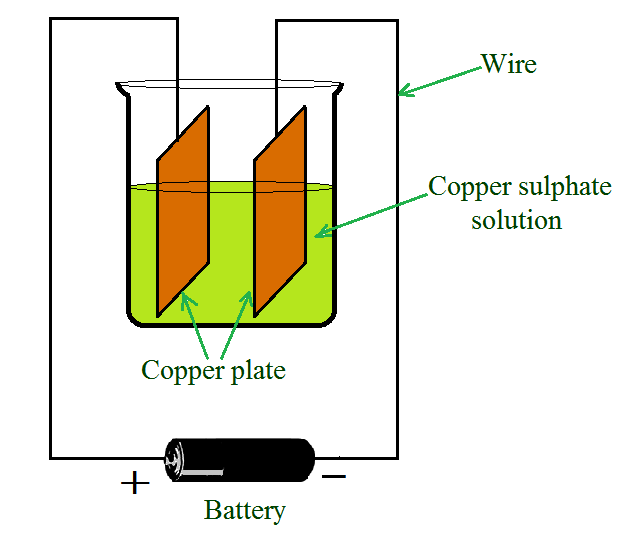
一个显示电镀的简单电路
让我们通过一个例子来了解电镀的过程:
- 当电流通过溶液时,硫酸铜分解成铜和硫酸盐。
- 游离铜被吸引到连接到电池负极端子的电极上并在其上沉积。
- 等量的铜溶解在来自另一个电极的溶液中。
- 结果,铜从溶液中流失。随着溶液中铜损失的恢复,该过程仍在进行中。
- 这意味着铜从一个电极转移到另一个电极。
电镀的过程是非常有益的。在工业中,它通常用于在金属物体上涂上一层薄薄的不同金属。沉积的金属层具有某种所需的特性,而物体的金属则不具备这些特性。例如,在汽车零件、浴室水龙头、厨房燃气灶、自行车车把、轮辋等许多物体上都进行了镀铬。它具有闪亮的外观,不腐蚀,并且抗划痕。
Some other applications of Electroplating are,
- To prevent corrosion and rust, zinc is applied to the iron.
- Silver and gold plating for jewelry.
- Tin is less reactive than iron, it is used to coat tin onto iron for cans.
示例问题
问题 1:当测试仪的自由端浸入溶液中时,磁针出现偏转。你能解释一下原因吗?
解决方案:
The effect of an electric current is considered to create a different type of tester. Regardless of whether the current is small, it is possible to observe a magnetic needle deflection. A tester is made by using the magnetic effect of current. The magnetic needle deflection indicates that the circuit is complete and that the solution conducts electricity, indicating that it is a good conductor.
问题 2:当测试仪的自由端浸入溶液中时,磁针出现偏转。你能解释一下原因吗?

解决方案:
Due to the following reasons, the bulb may not glow:
- A loose connection: A loose connection makes the circuit incomplete as it does not allow current to flow in the circuit.
- A fuse bulb is used: A fuse bulb does not glow as the filament gets broken.
- A discharged battery is used: A discharged battery doesn’t have power stored in it.
- The liquid used is distilled water: Distilled water is a poor conductor of electricity because it lacks dissolved salts that can provide it with ions needed to conduct electricity.
- A weak current is flowing in the circuit: A weak current does not have the power to heat the filament up to a level that makes then glow.
问题3:纯净水会导电吗?如果没有,我们能做些什么来让它导电?
解决方案:
No, pure water doesn’t conduct electricity. When salt is dissolved in pure water, it conducts electricity as it provide it with ions needed to conduct electricity.
问题4:什么是导体和绝缘体?
解决方案:
Conductors are materials that allow an electric current to flow through them. Materials that do not easily allow an electric current to flow through them, on the other hand, are poor conductors of electricity. Metals such as copper and aluminum have been discovered to carry electricity, whereas rubber, plastic, and wood do not.
问题5:什么是电镀?电镀的应用有哪些?
解决方案:
Electroplating is the process of depositing a layer of any desired metal on another material using electricity. It’s one of the most frequent uses of electric current’s chemical effects.
The procedure of electroplating is really beneficial. In industry, it’s commonly used to coat metal things with a thin layer of a different metal. The layer of metal deposited has some desired property, which the metal of the object doesn’t have. For example, chromium plating is done on many objects such as automobile parts, bath taps, kitchen gas burners, bicycle handlebars, wheel rims as well as numerous others. It has a shiny appearance, does not corrode, and resists scratches.
Some other applications of Electroplating are,
- To prevent corrosion and rust, zinc is applied to the iron.
- Silver and gold plating for jewelry.
- Tin is less reactive than iron, it is used to coat tin onto iron for cans.