丙酮配方——结构、性质、用途、示例问题
丙酮是一种无色液体,用于生产塑料和其他工业产品。丙酮分子式(或丙酮)在此以有机形式和结构形式给出,丙酮是最小和最简单的酮,它是一种易燃、无色和易挥发的液体。
丙酮配方
丙酮的化学式是C 3 H 6 O,也称为丙酮。丙酮存在于车辆、植物、树木和森林火灾的废气中。
它也存在于人体的尿液和血液中。丙酮与水、乙醚、乙醇混溶,具有刺激性、花香或刺激性气味。它被高度用作防腐剂和溶剂。炼金术士是第一个生产丙酮的人,它是通过金属醋酸盐的干馏生产的。它是由丙烯通过直接或间接方法生产的,或者我们可以说几乎 83% 的丙酮是在异丙苯过程中生产的。
丙酮的结构
这 丙酮的化学式由三个碳原子、六个氢原子和一个氧原子组成。它被认为是酮,因为其中存在羰基。主要用于医药和化妆品。丙酮主要存在于血液和尿液中。它也是指甲油去除剂中的活性成分。
Acetone Chemical Formula = C3H6O

丙酮结构 C 3 H 6 O 6
丙酮的制备
在工业中,83% 的丙酮是通过异丙苯工艺生产的。在此过程中,苯与丙烯烷基化生成异丙苯,异丙苯被空气氧化生成苯酚和丙酮。
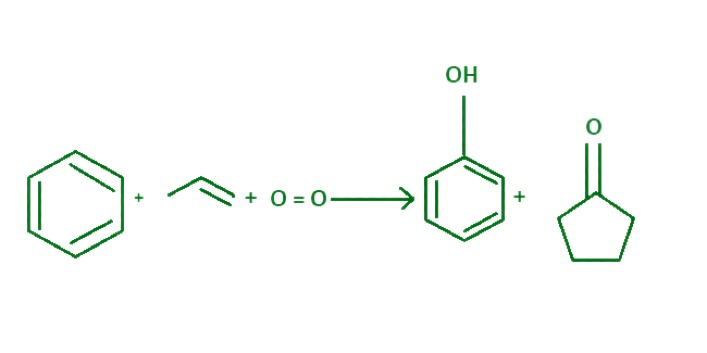
丙酮制备
发生
丙酮 C 3 H 6 O 天然存在于植物、树木、火山气体、森林火灾中,并且是身体脂肪分解的产物。它也存在于尿液和血液中,而糖尿病患者的浓度可能更高。我们可以在汽车尾气、烟草烟雾和垃圾填埋场找到它的踪迹。它也存在于木材、糖、纤维素等材料的分解蒸馏过程中形成的许多产品中。
丙酮的性质Chemical formula C3H6O Appearance of acetone Colorless liquid Odor of acetone Pungent, irritating, floral Boiling Point of acetone 56°C Melting Point of acetone -95°C Molecular mass of acetone 58.08 g/mol Density of acetone 0.7845 g/cm3 (25°C)
丙酮的化学性质
- 丙酮在水、乙醇、乙醚和甲醇中具有高度的混溶性。
- 它在室温下也是无色的,对热非常敏感,因此如果储存不当,丙酮在与空气或阳光接触时会迅速蒸发。
- 丙酮的另一个特性是它是一种非常有效的溶剂,可用于稀释多种稠密化学品。由于它具有高度挥发性,因此应远离火源和高温,以减少潜在的危险。
- 丙酮在环境中完全稳定。并且与水混合后化学性质会发生明显变化,在密闭容器中长期存放会因气体的积累而发生突然爆炸。
丙酮的用途
- 丙酮用作合成纤维和塑料的溶剂。
- 它用作甲基丙烯酸甲酯的前体。
- 它用于在涂漆前准备金属。
- 它用于制药工业中的一些药物。
- 它是挥发性的,因此在实验室中用于冲洗实验室玻璃器皿。
- 它用作干燥剂。
- 它用于脱脂过程。
- 用于洗甲水等化妆品。
- 它用于治疗痤疮。
概念问题
问题1:解释丙酮的用途?
回答:
Acetone is highly used as an industrial solvent. Acetone also serves as a precursor to methyl methacrylate. This process initiate with the conversion of acetone into acetone cyanohydrin.
It is a powerful solvent that is used as nail polish remover. Acetone is also effective for oil removal and preparing nail polish.
问题2:丙酮有害吗?
回答:
Intake of acetone from moderate to high amounts of acetone for a short amount of time can irritate your nose, throat, lungs and eyes and also cause headaches, a faster pulse. some other causes are nausea, effects on the blood, passing out and possible coma, and a shorter menstrual cycle in women.
问题 3:丙酮是比水更好的溶剂吗?
回答:
Acetone is a good solvent because it has the ability to dissolve both polar and nonpolar substances ,Second, because of its miscible substance. Acetone has the ability to mix with water in all proportions.
问题4:丙酮的化学名称和化学式是什么?丙酮在哪里出现?
回答:
The chemical formula of Acetone is C3H6O which is also known as propanone. It naturally occurs in plants, trees, volcanic gases, forest fires, and as a product of the breakdown of body fat. It is also found in urine and blood, while the concentration may be higher in diabetic patients. We can find the traces of it in vehicle exhaust, tobacco smoke, and landfill sites.
问题5:为什么丙酮比水重?
回答:
It is heavier because It exists in liquid form at room temperature, but it is less dense than about 1 g/mL of water at room temperature.
The density of acetone at room temperature is 0.788g/mL and which shows that per milliliter of liquid has a mass of 0.788 grams.
问题 6:丙酮对电子产品有何帮助?
回答:
It is highly combustible, on the other hand It is a important substance that has been used to clean electronic equipment and gadgets. It is work as a solvent in many of the electronic devices as well as work as a cleansers.
问题7:给出丙酮的两个优点和缺点?
回答:
Advantages of acetone are:
- Acetone is green biodegradable solvent.
- It has the ability to recycle by evaporation.
- It has higher extraction efficiency for xanthophylls.
Disadvantages of acetone are:
- It is a highly flammable
- It has very adverse effects on human health