磷酸铝配方 - 结构、性能、用途、示例问题
磷酸铝是一种化合物。它包含1个磷原子,4个氧原子,(3个与单键共用,1个与双键共用)和1个铝原子,通常显示为二水合物是指磷酸铝和2个水离子,五水合物是指磷酸铝和5个水离子。
磷酸铝在一般生活中有很多用途,现在让我们来看看什么是磷酸铝,它的结构、性能以及磷酸铝的用途。
什么是磷酸铝?
Aluminum phosphate is an amorphous form of aluminum hydroxy phosphate in this some of the hydroxyl groups of aluminum hydroxide are replaced by phosphate groups and it has many uses like flame retardant, Antacid, fungicide, catalyst food added substance, etc.
- The chemical formula of Aluminum Phosphate is AlPO4.
- It is generally occurring as an anhydrous salt and contains dihydrate and Pentahydrate structures.
- Dihydrate: AlPO4.2H2O
- Pentahydrate: AlPO4.5H2O
磷酸铝的结构
磷酸铝二水合物具有一组磷酸根阴离子、铝阳离子和水。它包含1个磷原子,4个氧原子,(3个与单键共用,1个与双键共用)和1个铝原子,通常显示为二水合物是指磷酸铝和2个水离子,五水合物是指磷酸铝和5个水离子。

磷酸铝的结构
磷酸铝的性质
磷酸铝的物理性质
- 磷酸铝是一种白色结晶粉末(水性无色液体),有恶臭)。
- 固态磷酸铝的密度为2.56 g.cm 3 。
- 磷酸铝的熔点为1800°C。
- 磷酸铝在其沸点分解。
- 磷酸铝不溶于水,极微溶于 HCl 和 HNO 3 。
- 磷酸铝的摩尔质量为 121.9529 g/mol,为白色结晶粉末。
- 磷酸铝的折射率为1.546。
磷酸铝化学性质
- 磷酸铝与盐酸 (HCL) 反应生成三氯化铝 (AlPO 4 ) 和磷酸 (H 3 PO 4 )。
AlPO 4 + 3HCl ⇢ AlCl 3 + H 3 PO 4
- 磷酸铝与氯化镁 (MgCl 2 ) 反应生成三氯化铝 (AlCl 3 ) 和磷酸镁 (Mg 3 (PO 4 ) 2 。
2AlPO 4 + 3MgCl 2 ⇢ Mg 3 (PO 4 ) 2 + 2AlCl 3
磷酸铝的用途
磷酸铝用于电子和电气企业、橡胶和粘合剂的制造、化学治疗药物的组装以及提高免疫原性的免疫接种。
此外,磷酸铝长期用作阻燃剂、抗酸剂、食品添加剂、杀菌剂、催化剂和白色着色剂、混凝土、牙科混凝土、腐蚀抑制剂。
示例问题
问题1:画出磷酸铝的结构。
回答:
Aluminum phosphate dihydrate has a group of tetra-and octahedral units of phosphate anions, aluminum cations, and water. Al3+ particles are 6-coordinate and PO43-particles are 4-coordinate.
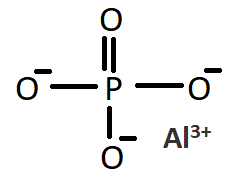
Structure of Aluminum Phosphate
问题二:磷酸铝的物理性质是什么?
回答:
Following are the Physical Properties of Aluminum Phosphate:
- Aluminum phosphate is a white crystalline powder (A colorless liquid in aqueous form) with a foul odour).
- The density of Aluminum phosphate is 2.56 g.cm3 in solid-state.
- The Melting point of Aluminum phosphate is 1800°C.
- The Aluminum phosphate is decomposed at its Boiling point.
- Aluminum phosphate is Insoluble in water and very slightly soluble in HCl & HNO3.
- The molar mass of Aluminum phosphate is 121.9529 g/mol as a white crystalline powder.
- The refractive index of Aluminum phosphate is 1.546.
问题3:磷酸铝的化学性质是什么?
回答:
- Aluminum phosphate reacts with Hydrochloric acid (HCL) and forms Aluminum trichloride (AlPO4) and Phosphoric acid (H3PO4).
AlPO4 + 3HCl ⇢ AlCl3 + H3PO4
- Aluminum phosphate reacts with Magnesium chloride (MgCl2) and forms Aluminum trichloride (AlCl3) and Magnesium phosphate (Mg3(PO4)2.
2AlPO4 + 3MgCl2 ⇢ Mg3(PO4)2 + 2AlCl3
问题四:磷酸铝有什么用途?
回答:
The uses of Aluminum Phosphate are:
- Aluminum phosphate is used in Electronic and Electrical ventures, manufacturing of rubber and Adhesive, assembling Chemotherapeutic medications, and immunizations to improve immunogenicity.
- Also, aluminum phosphate is used as a Flame retardant, Antacid, Food added substance, Fungicide, Catalyst, and a white colorant for a very long time, concrete, dental concrete, erosion inhibitors.
问题5:氯化镁与磷酸铝反应会发生什么?
回答:
Aluminum phosphate reacts with Magnesium chloride (MgCl2) and forms Aluminum trichloride (AlCl3) and Magnesium phosphate Mg3(PO4)2.
2AlPO4 + 3MgCl2 ⇢ Mg3(PO4)2 + 2AlCl3